Types of Chemical Reactions
What is a chemical reaction?
In chemistry, chemical reaction can be defined as:
“The process in which one or more substances (reactants) are combined chemically to form new substances (products) is known as a chemical reaction”
Generally, every element react as chemically and form bonds are called molecules. When molecules interact and change, chemical reactions take place.
The substances may be either chemical compounds or elements. During a chemical reaction, the constituent atoms of the reactants are rearranged to form different new substances as products:
-
Those chemical substances that react throughout a chemical reaction are called reactants.
-
Those chemical substances that are initiated during a chemical reaction are called products
Chemical reaction example:
Whether living organisms inhale oxygen from the surroundings which react with glucose and form carbon dioxide, water, and energy. The reaction is given as:
C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy
Characteristics of a Chemical Reaction:
Prior to discussing the types of chemical reactions, let’s look at the characteristics of chemical reactions that are described with the help of chemical reaction examples and mentioned below:
Evolution of Gas:
A reaction in which one of the products is a gas is evolved chemically is known as evolution of gas
Example:
When zinc reacts with hydrochloric acid, zinc chloride is formed by the evolution of hydrogen gas.
Reaction:
Zn + 2HCl → ZnCl2 + H2
Change in Colour:
A compound that changes colour during a chemical reaction that depends on the pH of the substance that is added to it. Since it is one of the types of chemical changes when the reaction proceeds chemically.
Example:
When colourless lead nitrate reacts with potassium iodide, the reaction forms a yellow precipitate of lead iodide and colourless potassium nitrate.
Reaction:
Pb(NO3)2 + 2KI → PbI2 + 2KNO3
Colourless Colourless Yellow ppt colourless
Formation of Precipitate:
During a chemical reaction, a precipitate is formed as a solid substance that is different from either of the reactants.
Example:
Barium chloride reacts with sodium sulphate and in products sodium chloride and precipitate of barium sulfate are formed.
Reaction:
BaCl2 + Na2SO4 —> BaSO4 + NaCl
Precipitate
Change in State:
A chemical reaction differ from physical changes due to the changes of states such as ice melting to water and water evaporating to vapor.
Example:
Ammonia gas reacts with hydrogen chloride gas and the reaction forms solid ammonium chloride crystals.
Reaction:
NH3(g) + HCl(g) —-> NH4Cl(s)
Change in Temperature:
A chemical reaction take place due to exothermic reaction as well as endothermic reaction.
Example:
Exothermic reactions:
During a chemical reaction, energy is released is termed as exothermic reaction.
Endothermic reactions:
During a chemical reaction, energy is absorbed is termed as endothermic reaction.
Change in Energy:
The energy change in a reaction is due to the difference in the amount of stored energy between the products and the reactants. During a chemical reaction, energy may be evolved or be absorbed.
Example:
Energy is released in combustion reactions, such as the burning of fuel in automobiles.
Moreover, you can also determine the moles of substances involved in chemical reactions easily by using the online
mole calculator.
6 Types of chemical reactions:
Here, we are discussing the most common different types of reactions chemistry. These include:
Combination Reaction:
"A chemical reaction in which two or more molecules (reactants) combine to form single compound (products) is termed a combination chemical reactions."
-
In general form: X + Y → XY
-
A combination reaction is also referred to as a synthesis reaction.
-
Example: 2Na + Cl2 → 2NaCl
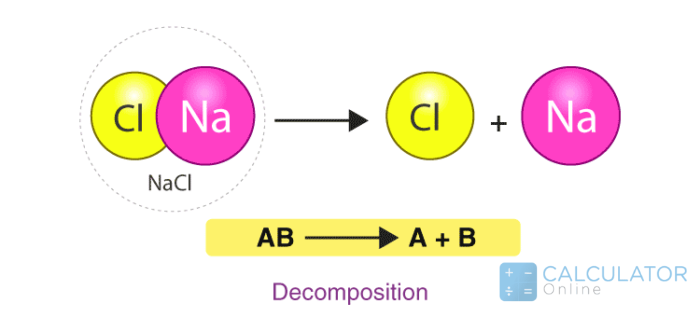
Decomposition reaction:
"A chemical reaction in which a molecule or compound breaks down into two or more compounds and forms simple substances is termed a decomposition reaction."
Displacement Reaction:
"It is one of the types of reactions in which a more reactive compound displaces a less reactive compound from its salt solution."
-
In general form: X + YZ → XZ + Y
-
It is also known as substitution reaction in chemistry
-
Example: Zn + CuSO4 → ZnSO4 + Cu
Double Displacement Reaction:
"A reaction in which two ionic compounds exchange their ions and form new two compounds is called a double displacement reaction."
-
Since it is also one of the all types of chemical reactions and let’s have a general form is given as:
-
XY + ZA → XZ + YA
-
It is also termed as a metathesis reaction
-
Example: KNO3 + AlCl3 ↔️ Al(NO3)3 + KCl
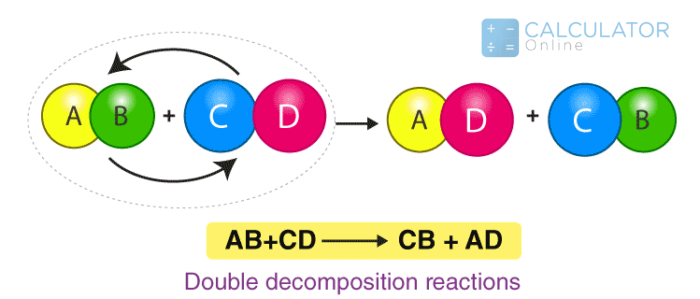
Precipitation Reaction or Double decompostion reactions:
"It also belongs to chemical reaction types in which two soluble salts in aqueous solutions combine and produce an insoluble precipitate."
-
In general form: XY + ZA → XA + YZ
-
Example: AgNO3(aq) + KCl(aq) ↔️ AgCl + KNO3(aq)
Neutralization reaction:
"A reaction in which an acid and a base react chemically and produce water and salts is known as a neutralization reaction."
-
Since it belongs to one of the different types of chemical reactions in chemistry and we have looks in general form.
-
XY + ZA → XZ + YA
-
The water molecule is formed by the mixture of OH– ions and H+ ions.
-
Example: NaOH + HCL → NaCL + H2O
Redox reactions:
The chemical reactions in which oxidation and reduction take place simultaneously is termed as redox reaction.
Oxidation reactions:
A reaction in which oxygen is added and hydrogen is removed in a compound is termed as oxidation reaction.
Reduction reactions:
A reaction in which hydrogen is added and oxygen is removed in compound is termed as reduction reaction
Example:
-
H2 + F2 → 2HF
-
H2 → H2+ 2e
-
F2 + 2e → 2F-
Besides, an online
redox reaction calculatorwill automatically indicate where the oxidation and reduction take place in the chemical reaction.
Chemical equation:
A chemical reaction is explained by a chemical equation, an general expression that gives the identities and quantities of the compound during a reaction. A chemical equation represent the occurring reactants (starting material) on the left and products (end results) on the right, separated by an arrow.
Skeleton Equation:
Each product that takes part in the chemical reaction is written in the form of chemical formulas and represent the chemical reaction is termed as skeleton equation.