Adblocker Detected

We always struggled to serve you with the best online calculations, thus, there's a humble request to either disable the AD blocker or go with premium plans to use the AD-Free version for calculators.
Disable your Adblocker and refresh your web page 😊
Table of Content
Employ the rate constant calculator to calculate the rate and concentration of the given substance over a specified period of time. This calculator makes it easy for you to find the constant rate and reaction rate.
Rate constant is known as the proportionality constant that shows the relation between the molar concentration of the reacting substance and the rate of reaction
Rate constant tells us how fast or slow a reaction is. It is affected by temperature and the activation energy. Higher temperature causes an increase in rate and lower temperature energy also causes an increase in rate constant.
The change in concentration of reactants and the products per unit of time is known as reaction rate. It is the quantification of how quickly a reaction takes place
This reaction rate can be affected by different factors including concentration, temperature, presence of a catalyst, and the surface area of a reactant.
kt = [R0] – [R]
k = ( [R0] – [R] ) / t
k = Rate constant.
[R0] = Initial concentration of the reactant (when t = 0)
[R] = Concentration of the reactant at time ‘t’
Suppose the initial concentration of A ([A0]) is 0.2 M, and after 30 minutes, the concentration ([A]) has decreased to 0.1 M. We want to calculate the rate constant (k) for this reaction.
As by the laws of the rate constant in this case the 1st order rate equation can apply.
[A] = [A]0 . e^-kt
Given Values:
Step # 1:
Substitute these values into the equation and solve for k
0.1 = 0.2 ⋅ e ^−k⋅30
Step # 2:
Divide both sides by 0.2
e ^−k⋅30 = 0.5
Step # 3:
Take the natural logarithm on both sides
−k ⋅ 30 = ln (0.5)
Step # 4:
Solve for k
k = − 30 / ln(0.5)
Calculate the numerical value for k and you’ll get the rate constant for this first-order reaction.
The rate of reaction calculator offers rate of reaction formulas for Zero Order, First Order, and Second Order reactions.
The rate of the reaction is independent of the concentration of the reactant is known as the zeroth-order reaction. In this law, the k remains constant regardless of the change in concentration.
Half-life = A / (2 × k)
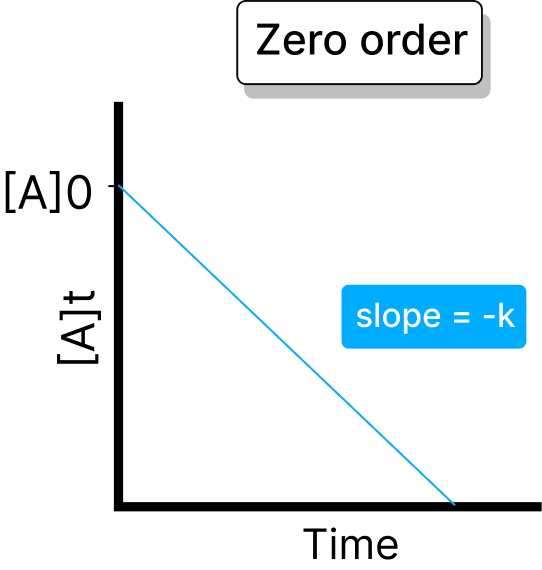
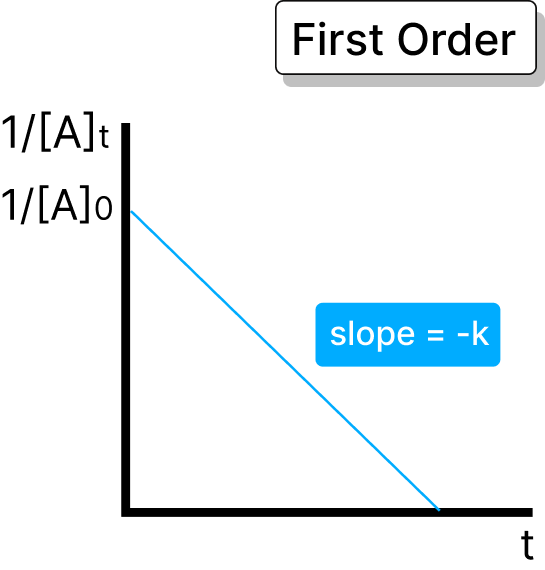
In this order, the rate of reaction is directly proportional to the concentration of the reactant. In this, the reaction rate increases with the increase in reactant concentration.
Half-life = 0.693 / k
Rate of the reaction = k × A
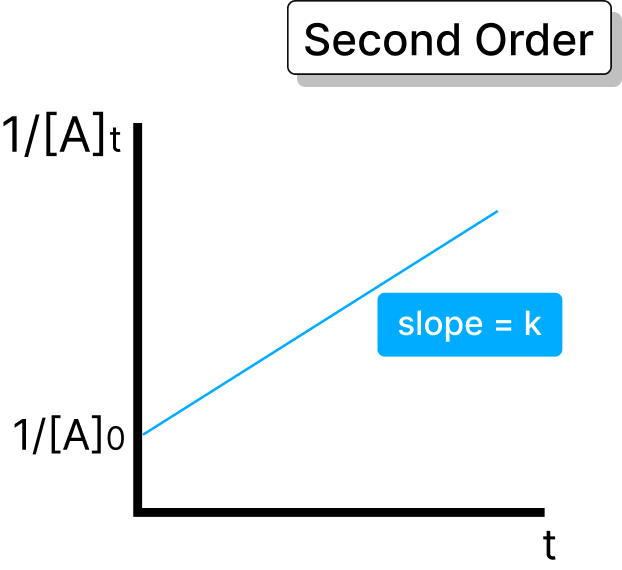
The rate is directly proportional to the square of the concentration of one reactant or the product of the concentration of two reactants. In this, the rate increases with the change in reactant concentrations.
Half-life = 1 / (k × A)
One substance: Rate of the reaction = k × A × A
Two substances: Rate of the reaction = k × A
Half-life T1/2 refers to the time that is taken for a substance to decrease by half. In radioactive decay, it is the time for half of a radioactive substance to decay into a more stable form. Different drugs have different half-lives and it takes 5 x to be considered cleared.
Unit of rate constant k = Unit of rate constant (k) = L mol -1 s. -1.
If the temperature goes high then the rate constant decreases and if the temperature decreases the rate constant decreases.
No, the rate constant can’t be negative because it quantifies the pace of concentration change over time.