Adblocker Detected

We always struggled to serve you with the best online calculations, thus, there's a humble request to either disable the AD blocker or go with premium plans to use the AD-Free version for calculators.
Disable your Adblocker and refresh your web page 😊
Table of Content
The ppm calculator measures the value of any quantity in the PPM(Part Per Millions), we can understand the PPM[mg/m3]. The PPM quantity is used to find the little amount of solute in a given amount of the Solution.
“1 ppm is equivalent or equal to the 1 milligram per liter of a substance(mg/L) or 1 milligram per Kilogram(mg/Kg)”.
Ppm is a way of expressing very dilute concentration of the substances.Just like the percent out of hundred, ppm means the part out of millions.
We are explaining how to measure ppm:
Consider:
1 Liter of water=1,000 cm^3=1Kg of water
Now consider
1cm^3=1g=1,000mm^3
1 Liter of water=1,000 cm^3=1,000,000 mm^3 of water
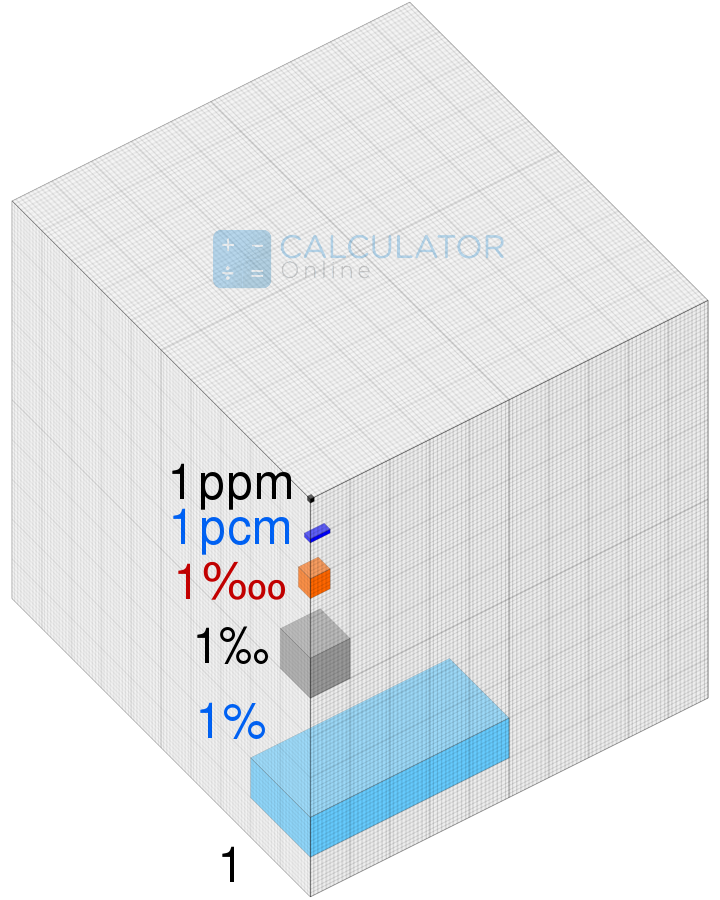
For example, if we are finding the impurity of Arsenic in 1L of water, we would define it as 1 PPM of Arsenic is present in 1L of water. The parts per million calculator finds the impurity of Arsenic in Per liter of water. The same way we can find the amount of lead(Pb), or Arsenic(Ar) impurity in the given amount of water.
In the figure below we have a 1 Liter of water present in a cube and find the Arsenic values in the 1L of water and it is represented by a small cube, which is exactly 1PPM.
We learn the PPM is a very small quantity and it is the part per million. We require the part per million in a large amount of solutions. Using the term ppm we can express the concentration as a very small fraction of larger quantity. For example we are finding the quantity of Arsenic in a Pool of water, the quantity would be as ppm or milligrams/Liter (mg/L).
It means 1 liter of water containing 1 millions milligram or 1,000,000 mm^3
So we have:
1,000,000 mm^3 =mg/L=1 ppm
The ppm calculation finds the concentration of the solute ein in the solution.
We need to cover the PPM to mg/m3 for gasses, it can be easy to convert the PPM to mg/m3 by the parts per million calculator.
The formula for the conversion:
concentration (mg/m3) = 0.0409 x concentration (ppm) x molecular weight
Example:
Convert 100 ppm of NH3 into the mg/m3:
Sol:
Now the molecular weight of NH3=17.03 g/mol
concentration (mg/m3) = 0.0409 x concentration (ppm) x molecular weight
Concentration of NH3 (mg/m3)=0.0409 x 100 ppm x 17.03 = 69.652 (mg/m3)
We are presenting a table conversion of 100 PPM of gasses into (mg/m3).
| Gasses | Output (mg/m3) | Molecular weight |
| (Ammonia)NH3 | 69.652 mg/m3 | M = 17.03 g/mol |
| Carbon monoxide [CO] | 114.561 mg/m3 | M = 28.01 g/mol |
| Carbon dioxide [CO2] | 180.001 mg/m3 | M = 44.01 g/mol |
| Chlorine [Cl2] | 289.981 mg/m3 | M = 70.9 g/mol |
| Formaldehyde [CH2O] | 122.806 mg/m3 | M = 30.026 g/mol |
| Hydrogen [H2] | 8.262 mg/m3 | M = 2.02 g/mol |
| Methane [CH4] | 65.604 mg/m3 | M = 16.04 g/mol |
| Hydrogen Sulfide [H2S] | 139.387 mg/m3 | M = 34.08 g/mol |
| Nitrogen Dioxide [NO2] | 188.181 mg/m3 | M = 46.01 g/mol |
| Ozone [O3] | 196.320 mg/m3 | M = 48.00 g/mol |
The concentration to ppm calculator converts the 100 PPM of gasses into the mg/m3.
There are various applications of the ppm formula in the field chemistry especially we are dealing with the impurities
We do use the ppm calculation chart as represented in the following table,we also define other units like (part per billion) ppb(10^9) and smallest units like(part per trillion)ppt(10^12).
The ppm calculator finds the ppm,ppb,and ppt values of the following table.
| 1 of →
= ⭨ of ↓ |
per
cent (%) |
per
1,000 (‰) |
per
10,000 (‱) |
per
100,000 (pcm) |
per
million (ppm) |
per
billion (ppb) |
| % | 1 | 0.1 | 0.01 | 0.001 | 0.0001 | 10−7 |
| ‰ | 10 | 1 | 0.1 | 0.01 | 0.001 | 10−6 |
| ‱ | 100 | 10 | 1 | 0.1 | 0.01 | 10−5 |
| pcm | 1,000 | 100 | 10 | 1 | 0.1 | 0.0001 |
| ppm | 10,000 | 1,000 | 100 | 10 | 1 | 0.001 |
| ppb | 107 | 106 | 105 | 10,000 | 1,000 | 1 |
Exp1:
A solution contains 5 g of Arsenic in 1500 grams of solution. Find the solution concentration in ppm?
Sol:
ppm= [Grams of the Solute/Grams of the Solution]X 1,000,000
ppm=[5g/1500g]X 1,000,000
The quantity of Arsenic =3333.33 ppm
Or
The quantity of Arsenic =3333ppm
We can find the ppm calculation by the ppm calculator.
Exp2:
How many grams of solute are dissolved in 100 grams of 1.2X 10^2 ppm solution?
Solution:
ppm= [Grams of the Solute/Grams of the Solution]X 1,000,000
1.2X 10^2= [Grams of the Solute/100g]X 1,000,000
Grams of the Solute=[1.2X 10^2X100]/1,000,000
Grams of the Solute=0.012 g
It means 0.012 g of solute are present in the solution, ppm converter can find the solute concentration.
The ppm is one of the most tiniest of the calculation we are using in the chemical analysis, we can use the ppm concentration calculator to measure it :
Input:
Output:
The ppm converter is readily able to find the molar concentration of any impurity in a given quantity of solution.
0.0001%, the 1 ppm=1/1,000,000 as 0.000001 or 0.0001%, , we can use the percent to ppm calculator to find the %age of impurity.
We can find both ppm by mass or volume.
ppm by mass:
ppm by mass is equivalent to milligrams per liter (mg/L).
ppm by volume:
ppm by volume is a common way to measure the concentrations of gasses.
The ppb and ppt are smaller than ppm as the ppb=10^9, and ppt=10^12 but the ppm=10^6. The ppb and ppt values are evaluated by the ppm calculator
The ppm calculation is just too important when we are dealing with the little quantities of the impurities in the given solution. It helps to make the solution pure from impurities or keep the level to our desire. We need an especially designed ppm calculator to find the values in the given volume of the solution.
From the source of the Wikipedia:Parts-per notation,Parts-per expressions
From the source of the greenfacts.org:Parts per million,Definition