Adblocker Detected

We always struggled to serve you with the best online calculations, thus, there's a humble request to either disable the AD blocker or go with premium plans to use the AD-Free version for calculators.
Disable your Adblocker and refresh your web page 😊
Table of Content
The free online combined gas law calculator displays, either the temperature, pressure, or volume of the gasses with the help of the combined gas law.
The combined gas law calculations make the calculations faster, efficient and display the unknown values in a matter of a fraction of a second.
“The combined gas law combines the three gas laws: Boyle’s Law, Charles’ Law, and Gay-Lussac’s Law. It states that the ratio of the product of pressure and volume and the absolute temperature of a gas is equal to a constant”
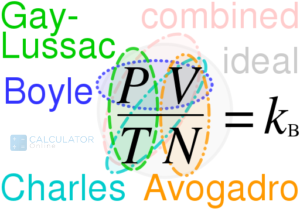
The simple combined gas law equation:
PV/T = k
Where:
k= Constant of proportionality
P=Pressure of the gasses
V= Volume of the gasses
T= Temperature of the gasses.
When we need to compute the changes in temperature, volume, and pressure of the sample gas. We can write the combined gas law formula as:
P1V1/T1=P2V2/T2
There are various units of the volume, pressure, and temperature, we can use to input in the combined gas law calculator. This calculator supports the various measurements in standard units of the volume temperature and the pressure.
By default unit of the volume in the gas volume calculator is m^3. We can find the vloume of the gas by rhte volume of gas calculator in a matter of seconds.We can find the the other conversion of units to m3(meter cube) by the gas volume calculator.
| From | To: m3 |
| l | 0.001 |
| ml | 0.000001 |
| ft^3 | 0.02831685 |
| in^3 | 0.00001638706 |
By default is the unit of the pressure of Pascal and it can be coveint to find the otehr units of the pressure by the pressure calculator chemistry. We are giving the conversion of remaining units of the pressure into the Pascal in the below table:
| From: | To: Pa |
| KPa | 1000 |
| Bar | 100000 |
| atm | 101325.01 |
| mmHg | 133.32238157895 |
| mbar | 100 |
The combined gas law calculator using Kelvin as a unit of temperature. You can specify the various units like the Celsius and the Fahrenheit as per your wish. The conversion from Celsius and Fahrenheit to Kelvin is given below:
From °C to K = t°C + 273.15
From °F to K = (t°F + 459.67) * 5/9
The combined gas law is a combination or of three of the known gas laws
It states the ratio of pressure, volumes and the absolute temperature is always equal law, thenonstant. When we combine Avogadro’s Law with the combined gas law then we are able to define the ideal gas law. This is the main reason there are no official discoveries of the Combined gas law as it is just a combination of all the gas-known laws. The combined gas law formula calculator using all the known laws of the gases and to implement the combined gas law.
Consider an initial volume of a gas is 6 liters and the final volume is 3 liters. Find out the final pressure of the gas if the initial temperature was 273 K and the final temperature is 200 K. The initial pressure was 25 K Pa.
Sol:
P1=25 K Pa
V1=6 L
V2= 3L
T1=273 K
T2=200K
We can find the final pressure by the combined law calculator in amatter of minutes.
Now by applying the combined gas law formula:
P1V1/T1 = P2V2/T2
25 × 6/273 = P2 × 3/200
P2 = 36.626 KPa
Hence the final pressure=P2= = 36.626 KPa of the gas. We can check the answer with the combined gas laws calculator.
The volume temperature and pressure calculator measure accurately either the pressure, temperature, or the volume of any gas by combining the Charles, Boyle, and Gay-Lussac laws:
Input:
Output:
The combined gas law calculator displays following output
A Pascal is the unit of the pressure and is defined as the “One pascal is a pressure of one newton per square meter”. The kPa standard “atm” pressurealso used along with the pascal.
The standard atmospheric pressure is abbreviated as standard atm.
1 atm=101,325 pascal
The 1 torr= 1mmHG of mercury at “0” Centigrade. The name of torr was given by the Torricelli, a person who discovered this unit of pressure.
The conversion of mmHg can be found by the following relations
The combined gas law calculator with steps using most of the common units of the pressure.
A bar is a metric unit of pressure and it is equal to 100,00 Pascals.
1 bar =100,00 pascals
1 bar =0.987 atmospheres
A kelvin is a unit of temperature and is less commonly known as the degree Kelvin. It is a standard SI unit of temperature and one Kelvin is defined as the 1/273.16 (3.6609 x 10 -3 ) of the thermodynamic temperature of pure water (H_2O)
The combined gas law is vastly used in thermodynamics and fluid mechanics. The other gas laws have lower applications as we maintain the constant value of the remaining variables. This is the main reason we are using the combined gas laws most commonly in finding the temperature, pressure, and volume of the gasses. We can use the combined gas law calculator to find the final values by the combined gas law calculations.
From the source of Khan Academy:The ideal gas law,calculate number of moles
From the source of Wikipedia:Combined gas law,Derivation from the gas laws