Adblocker Detected

We always struggled to serve you with the best online calculations, thus, there's a humble request to either disable the AD blocker or go with premium plans to use the AD-Free version for calculators.
Disable your Adblocker and refresh your web page 😊
An online condensed electron configuration calculator helps you to determine the electron configuration of any element. This valence electron calculator displays the abbreviated configuration and the atomic number of each element. Read on to understand abbreviated electron configuration, shells, subshell, and how to find electron configuration of an atom or element.
In quantum chemistry and atomic physics, the electron configuration of an atom or molecule describes the distribution of electron distribution mnemonics in different atomic or molecular orbitals. It also describes every electron as moving freely in an orbital, in an average field generated by other orbitals. For example, electron configuration of Phosphorus (P) is 1s^2 2s^2 2p^6 3s^2 3p^3.
Usually, Physicists and chemists use the isotope notation calculator to refer how to calculate electronic configurations of molecules and atoms. For atoms, the standard notation consists of a series of atomic subshell labels (for example, phosphorus sequence of notation is 1s, 2s, 2p, 3s, 3p), where the number of electrons assigned to each subshell is used as a superscript. For example, hydrogen has just 1 electron in the s orbital of the first shell, so its electron configuration notation is recorded as 1s^1. Lithium has two electrons in the 1s subshell and one electron in the 2s subshell (higher energy), so its electron configuration is 1s^2 and 2s^1. Rest for verification, keep using this electron configuration generator.
Apart from this, Electron Configuration:
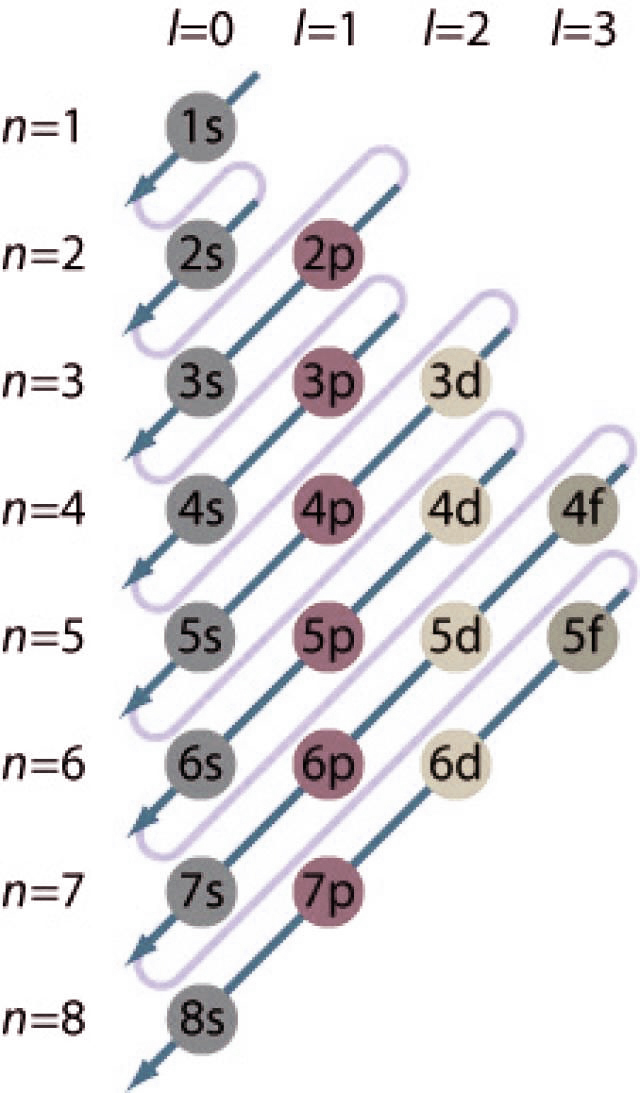
Our ground state electron configuration calculator with charges also depicts an abbreviated way of finding electron configuration. If you want to do manually then follow the steps below to write shorthand electron configurations:
However, an Online Angular Velocity Calculator allows you to determine the angular velocity of the body in motion on a circular path.
Electron shells are a set of feasible states that have the same principal quantum number n (the number before the letter on the orbital) that the electron can occupy. An atom with an nth electron shell can hold 2n^2 electrons, which is the first shell that can hold 2 electrons, the second shell can hold 8 electrons, and so on.
A subshell is a set of states, which are defined by the total azimuth quantum number “l” in the shell. The range of ℓ value is 0 to n-1. The values of ℓ = 0, 1, 2, and 3 correspond to the orbit s, p, d, and f, respectively.
It is believed that the atomic mass of an element is closely related to the atomic number, because if the atomic mass is high, then the atomic number is also high, what is the difference between atomic mass and atomic number? To find out, let’s look at some key differences.
| Atomic Mass | Atomic Number |
| Atomic mass is related to the number of neutrons and protons present in a particular nucleus of an element. | The atomic number is usually the number of protons present in the nucleus of an element that you can also determine using this best atomic number calculator. |
| It is the average weight of an atom or molecule. | It is the total number of nucleons in the atom’s nucleus. |
| Atomic mass is always denoted by A | The atomic number is always denoted by Z |
| The atomic mass unit (AMU) is usually used to measure atomic mass. | An atomic number is a number that is used to place elements in the periodic table. |
However, an Online Photon Energy Calculator will allow you to find the energy of a photon from its wavelength & frequency.
An online valence electrons calculator finds the abbreviated or condensed electron configuration of an element with these instructions:
There are three important rules used for electron configuration: Pauli-exclusion Principle, Aufbau Principle, and Hund’s Rule (The one that is also elaborated by this hund’s rule calculator). And when it comes to understanding the basic theory behind all these principles, this online electron configuration finder will help you sort the ways to understand the scenario better.
K represents the first level of energy, L represents the second level of energy, M represents the third level of energy in an atom or molecule, and so on. In other words, the symbol KLMN only represents the number of electrons in an atom. And this free electron configuration calculator is designed to arrange the number of atoms in these shells accordingly.
The mass of an electron is much smaller than a proton with only 9.11 x 10^{-28} grams or about 1/1800 atomic mass units. Therefore, they do not increase or decrease the total atomic mass of the element.
The following sentence aids you a lot in remembering the first 6 elements of the periodic table.
“Happy Henry Lives Beside Boron Cottage”
Where :
Happy = Hydrogen
Henry = Helium
Lives = Lithium
Beside = Beryllium
Boron = Boron
Cottage = Carbon
Moreover when it comes to the configuration of these, this electronic configuration calculator is the one that stands out by providing you to the point orbital notation for each atom.
An online noble gas electron configuration calculator provides a condensed method of finding the electron configuration, atomic number, and atomic mass of given. Yes, this free orbital diagram calculator can quickly and easily tell the reader how many electron orbitals an atom has, and how many electrons there are in each atom.
From the source of Wikipedia: Shells and subshells, Notation, Energy—ground state and excited states.
From the source of Science Notes: Electron Configurations of Elements, How to Find Electron Configuration.
From the source of Chemistry Edu: Electron Configuration, Order of Fill, How to Write an Electron Configuration, Special Cases, Exceptions, Periodic Properties.